Rheumatoid arthritis (RA) has a typical onset in middle age with a stronger predominance in women. Testing for serum RF and anticyclic citrullinated peptide (CCP) antibodies, a test with higher specificity, may help corroborate or provide a harbinger for clinical diagnosis of RA. It should be noted that serum RF may not be positive in the early stages of RA, in contrast to the anti-CCP antibody, which may predate full-blown RA by months to years.
In regards to the differential diagnosis of RA, various forms of viral arthritis, most notably, arthritis due to parvovirus B19, hepatitis B (in the anicteric phase), hepatitis C, and human immunodeficiency virus (HIV), can present with sudden onset joint pain and/or swelling.
Early disease modifying antirheumatic drug (DMARD) therapy in RA substantially improves signs and symptoms of the disease, limits joint damage, and improves outcomes and long-term prognosis. Methotrexate is the most common first DMARD initiated for RA, and a typical starting dose is between 7.5 to 15 mg weekly. Methotrexate is optimally administered with daily folic acid supplementation. While nonsteroidal anti-inflammatory drugs (NSAIDs), like diclofenac, may provide relief of signs and symptoms, they do not improve outcomes or prognosis in RA.
Disease modifying antirheumatic drug therapy should be started early, ideally within 3 months from the onset of symptoms. The most commonly initiated first DMARD for RA is methotrexate. With the initiation of methotrexate, patients should have baseline liver enzymes and a complete blood count (CBC), and these tests should be monitored at regular intervals to exclude hematologic or hepatic toxicity attributable to methotrexate.
The 2012 ACR recommendations suggest to measure RA disease activity in a quantitative way, and to therapeutically aim for a target of remission or low disease activity; however the recommendations do not specify which specific measurement tools to use.
The RAPID3 (Routine Assessment of Patient Index Data 3 ),
CDAI (Clinical Disease Activity Index),
DAS28 (Disease Activity Score in 28 joints with ESR), and
SDAI (Simplified Disease Activity Index)
are all mentioned in the ACR guidelines, however use of any of these measures as well as the HAQ-DI (Health Assessment Questionnaire Disability Index) are appropriate to use as long as they are used consistently to follow RA disease activity over time.
2012 ACR Recommendations for the Treatment of Early RA (<6 Months)
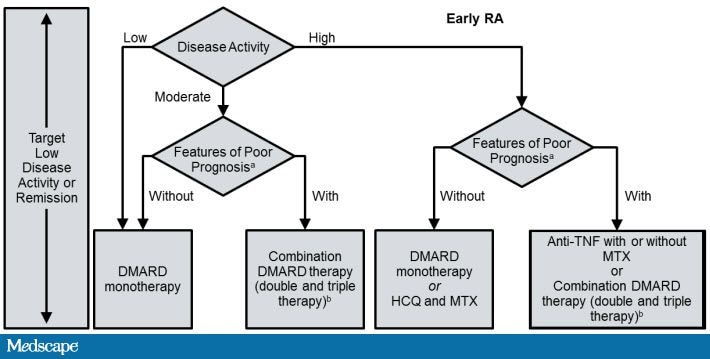
ACR = American College of Rheumatology; CCP = cyclic citrullinated peptide; DMARD = disease modifying antirheumatic drug; HAQ-DI = Health Assessment Questionnaire-Disability Index; HCQ = hydroxychloroquine; LEF = leflunomide; MTX = methotrexate; RA = rheumatoid arthritis; TNF = tumor necrosis factor.
aPatients were categorized based on the presence or absence of 1 or more of the following poor prognostic features: functional limitation (eg, HAQ-DI or similar validated tools), extra-articular disease (eg, presence of rheumatoid nodules, RA vasculitis, Felty’s syndrome), positive rheumatoid factor or anti-CCP antibodies, and bony erosions by radiograph.
bCombination DMARD therapy with 2 DMARDs, which is most commonly MTX-based with some exceptions (eg, MTX+HCQ, MTX+LEF, MTX+sulfasalazine, and sulfasalazine+HCQ), and triple therapy (MTX+HCQ+sulfasalazine).
The majority of the clinical trials with the anti-TNF agents have examined patients who had previously failed treatment with nonbiologic DMARDs, where biologic agents were added to methotrexate. These studies have consistently demonstrated, largely across the different anti-TNF agents, that anti-TNF agents have superior efficacy in terms of disease activity and retarding progression of radiographic damage (bone erosions and joint space narrowing) compared to the use of methotrexate alone.
Anti-TNF agents have been associated with a heightened risk for both typical and atypical infections based on both clinical trials and large observational studies. The 2012 ACR guidelines recommend the use of either a TB skin test (purified protein derivative [PPD]) or an interferon gamma release assay (IGRA), commonly called a quantiferon test, for TB screening prior to use of biologic agents.
The IGRA has a particular advantage in patients who have been vaccinated in the past with the Bacillus Calmette-Guérin vaccine.
Evidence has not shown increased efficacy for doubling the frequency of dosing for anti-TNF therapy (i.e if patient having moderate disease activity even with alternate weekly dose of adalimumab 40) and the combination of biologics has been associated with unacceptable toxicity. According to the guidelines, this patient should be switched to either a different anti-TNF agent or to a non-TNF biologic agent (abatacept, rituximab, or tocilizumab)
2012 ACR Recommendations for the Treatment of Established RA Disease (Duration ≥6 Months).
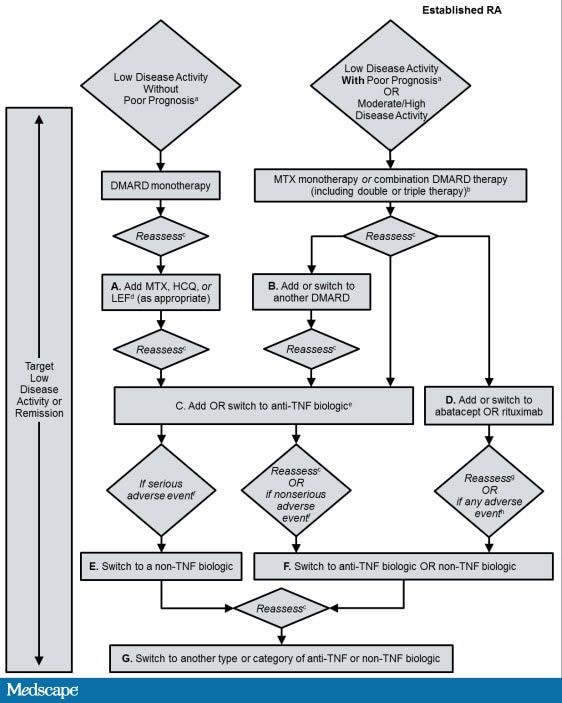
There have been several studies that have examined the additive use of biologic agents and while there may be some synergistic benefit in terms of efficacy, the toxicity, particularly from infections, has been considered unacceptable for the use of combinations of biologic agents. Thus, combination biologic therapy (eg, abatacept plus an anti-TNF agent ) is not recommended based upon the available evidence.
The 2012 ACR guidelines recommend against administering live virus vaccinations including influenza vaccine administered by nasal spray, herpes zoster vaccination, and yellow fever vaccine in patients currently on biologic therapy. Inactivated vaccines including injectable influenza and pneumococcal vaccinations may safely be given to patients receiving biologic agents and nonbiologic DMARDs.
2012 ACR recommendations Regarding the Use of Vaccines in Patients With RA Starting or Currently Receiving DMARDs or Biologic Agents
In regards to the differential diagnosis of RA, various forms of viral arthritis, most notably, arthritis due to parvovirus B19, hepatitis B (in the anicteric phase), hepatitis C, and human immunodeficiency virus (HIV), can present with sudden onset joint pain and/or swelling.
Early disease modifying antirheumatic drug (DMARD) therapy in RA substantially improves signs and symptoms of the disease, limits joint damage, and improves outcomes and long-term prognosis. Methotrexate is the most common first DMARD initiated for RA, and a typical starting dose is between 7.5 to 15 mg weekly. Methotrexate is optimally administered with daily folic acid supplementation. While nonsteroidal anti-inflammatory drugs (NSAIDs), like diclofenac, may provide relief of signs and symptoms, they do not improve outcomes or prognosis in RA.
Disease modifying antirheumatic drug therapy should be started early, ideally within 3 months from the onset of symptoms. The most commonly initiated first DMARD for RA is methotrexate. With the initiation of methotrexate, patients should have baseline liver enzymes and a complete blood count (CBC), and these tests should be monitored at regular intervals to exclude hematologic or hepatic toxicity attributable to methotrexate.
The 2012 ACR recommendations suggest to measure RA disease activity in a quantitative way, and to therapeutically aim for a target of remission or low disease activity; however the recommendations do not specify which specific measurement tools to use.
The RAPID3 (Routine Assessment of Patient Index Data 3 ),
CDAI (Clinical Disease Activity Index),
DAS28 (Disease Activity Score in 28 joints with ESR), and
SDAI (Simplified Disease Activity Index)
are all mentioned in the ACR guidelines, however use of any of these measures as well as the HAQ-DI (Health Assessment Questionnaire Disability Index) are appropriate to use as long as they are used consistently to follow RA disease activity over time.
2012 ACR Recommendations for the Treatment of Early RA (<6 Months)

ACR = American College of Rheumatology; CCP = cyclic citrullinated peptide; DMARD = disease modifying antirheumatic drug; HAQ-DI = Health Assessment Questionnaire-Disability Index; HCQ = hydroxychloroquine; LEF = leflunomide; MTX = methotrexate; RA = rheumatoid arthritis; TNF = tumor necrosis factor.
aPatients were categorized based on the presence or absence of 1 or more of the following poor prognostic features: functional limitation (eg, HAQ-DI or similar validated tools), extra-articular disease (eg, presence of rheumatoid nodules, RA vasculitis, Felty’s syndrome), positive rheumatoid factor or anti-CCP antibodies, and bony erosions by radiograph.
bCombination DMARD therapy with 2 DMARDs, which is most commonly MTX-based with some exceptions (eg, MTX+HCQ, MTX+LEF, MTX+sulfasalazine, and sulfasalazine+HCQ), and triple therapy (MTX+HCQ+sulfasalazine).
The majority of the clinical trials with the anti-TNF agents have examined patients who had previously failed treatment with nonbiologic DMARDs, where biologic agents were added to methotrexate. These studies have consistently demonstrated, largely across the different anti-TNF agents, that anti-TNF agents have superior efficacy in terms of disease activity and retarding progression of radiographic damage (bone erosions and joint space narrowing) compared to the use of methotrexate alone.
Anti-TNF agents have been associated with a heightened risk for both typical and atypical infections based on both clinical trials and large observational studies. The 2012 ACR guidelines recommend the use of either a TB skin test (purified protein derivative [PPD]) or an interferon gamma release assay (IGRA), commonly called a quantiferon test, for TB screening prior to use of biologic agents.
The IGRA has a particular advantage in patients who have been vaccinated in the past with the Bacillus Calmette-Guérin vaccine.
Evidence has not shown increased efficacy for doubling the frequency of dosing for anti-TNF therapy (i.e if patient having moderate disease activity even with alternate weekly dose of adalimumab 40) and the combination of biologics has been associated with unacceptable toxicity. According to the guidelines, this patient should be switched to either a different anti-TNF agent or to a non-TNF biologic agent (abatacept, rituximab, or tocilizumab)
2012 ACR Recommendations for the Treatment of Established RA Disease (Duration ≥6 Months).

There have been several studies that have examined the additive use of biologic agents and while there may be some synergistic benefit in terms of efficacy, the toxicity, particularly from infections, has been considered unacceptable for the use of combinations of biologic agents. Thus, combination biologic therapy (eg, abatacept plus an anti-TNF agent ) is not recommended based upon the available evidence.
The 2012 ACR guidelines recommend against administering live virus vaccinations including influenza vaccine administered by nasal spray, herpes zoster vaccination, and yellow fever vaccine in patients currently on biologic therapy. Inactivated vaccines including injectable influenza and pneumococcal vaccinations may safely be given to patients receiving biologic agents and nonbiologic DMARDs.
2012 ACR recommendations Regarding the Use of Vaccines in Patients With RA Starting or Currently Receiving DMARDs or Biologic Agents
| Killed Vaccines | Recombinant Vaccine | Live Attenuated Vaccine | |||
|---|---|---|---|---|---|
| Pneumococcala | Influenza (intramuscular) | Hepatitis Bb | Human papillomavirus | Herpes zoster | |
| Before initiating therapy: | |||||
| DMARD monotherapy | √ | √ | √ | √ | √ |
| Combination DMARDs | √ | √ | √ | √ | √ |
| Anti-TNF biologics | √ | √ | √ | √ | √ |
| Non-TNF biologics | √ | √ | √ | √ | √ |
| While already taking therapy: | |||||
| DMARD monotherapy | √ | √ | √ | √ | √ |
| Combination DMARDs | √ | √ | √ | √ | √ |
| Anti-TNF biologics | √ | √ | √ | √ | Not recommended |
| Non-TNF biologics | √ | √ | √ | √ | Not recommended |
No comments:
Post a Comment